Oleander Hawkmoth
| Photograph by Melissa Teo |
Moths never looked this good...
Table of Contents
Synonyms:
Common NameArmy Green Hawkmoth
Scientific Name
Sphinx nerii
Deilephila nerii (erronous)
Histriosphinx nerii
Etymology:
Common Name“Oleander” in the “Oleander Hawkmoth” refers to the host plant it feeds on in the larval stage, Nerium oleander (family Apocynaceae) [1]. It is also sometimes referred to as the Army Green Hawkmoth for its foliage-like colours and patterns.
Scientific Name
Daphnis: Likely a reference to Daphnis, son of Hermes and a Nymphe in Greek mythology. He was said to be good at playing the pipes and was exceedingly beautiful [2].
This moth was first described by Linnaeus, 1758, in the first volume of Systema Naturae as Sphinx nerii.
Histriosphinx Varis, 1976, Notul. ent. 56: 127.Transferred to Daphnis:Hübner, (1819) Verz. Bekannt. Schmett.: 134
Deiliphila Zhu & Wang, 1997, Fauna Sinica Insecta 11: 290 (key), 292. [3]
Biology:
Ø Diet
Despite its common name, the larvae of D. nerii also feed on non-oleander plants of the same family. Other possible host plants include up to 32 species from Rubiaceae and Apocynaceae [4]. |
| © Photograph by A. R. Pittaway |
Fig. 1. Nerium oleander
List of other host plants [4]
- Anacardiaceae (cashew): Mangifera (related to mango)
- Apocynaceae (Dogbane; mainly tropical):Vinca (periwinkle), Vitis (grapevines), Asclepias (milkweed), Trachelospermum (woody vines), Amsonia (bluestars), Carissa (related to conkerberry), Tabernaemontana (milkwood), Rhazya, Adenium (common bonsai plant), Catharanthus (periwinkle), Thevetia (oleander)
- Convolvulaceae (morning glory): Ipomoea (morning glory)
- Oleaceae (related to Olive plants): Jasminum (jasmine), Ligustrum ovalifolium (privet)
- Rubiaceae (related to Coffee plants): Gardenia (gardenias)
Adults are non-specific and will nectar from a variety of flowers. Specimens in captivity will also drink sucrose solution.
Ø Life history and behaviour
As with all Lepidopterans, D. nerii is holometabolous and undergoes a four stage lifecycle in which each stage shows a significantly different morphology.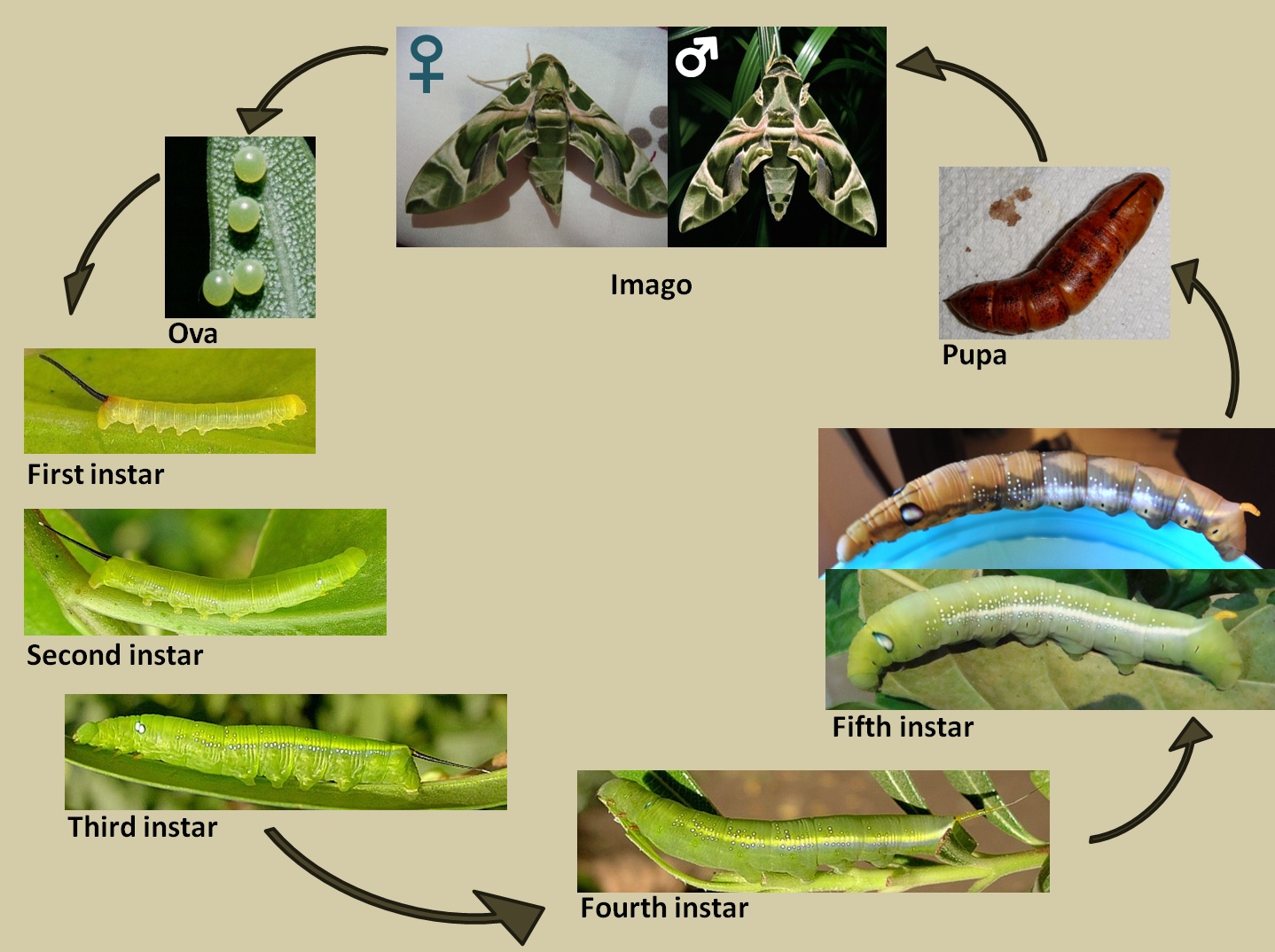 |
| Ova to fourth instar, and imago male © photograph by A. R. Pittaway; fifth instar to imago female photograph by Melissa Teo |
Fig. 2. Life stages of D. nerii.
As main function of the larval stage is to obtain as much nutrient as possible before metamorphosis into an adult, and the main food source of the larvae is sessile, they are relatively immobile and slow-moving. Hence, much of their defence comes from the toxin in the sap of N. oleander which they feed on [5].
Video 1. Feeding behaviour of D. nerii caterpillar. Only the head and rear end make significant movement.
When provoked, the caterpillar first straightens itself out to resemble and oleander leaf. If the threat continues, it curls its upper segments to display the prominent eye-spots on the third thoracic segment [1].
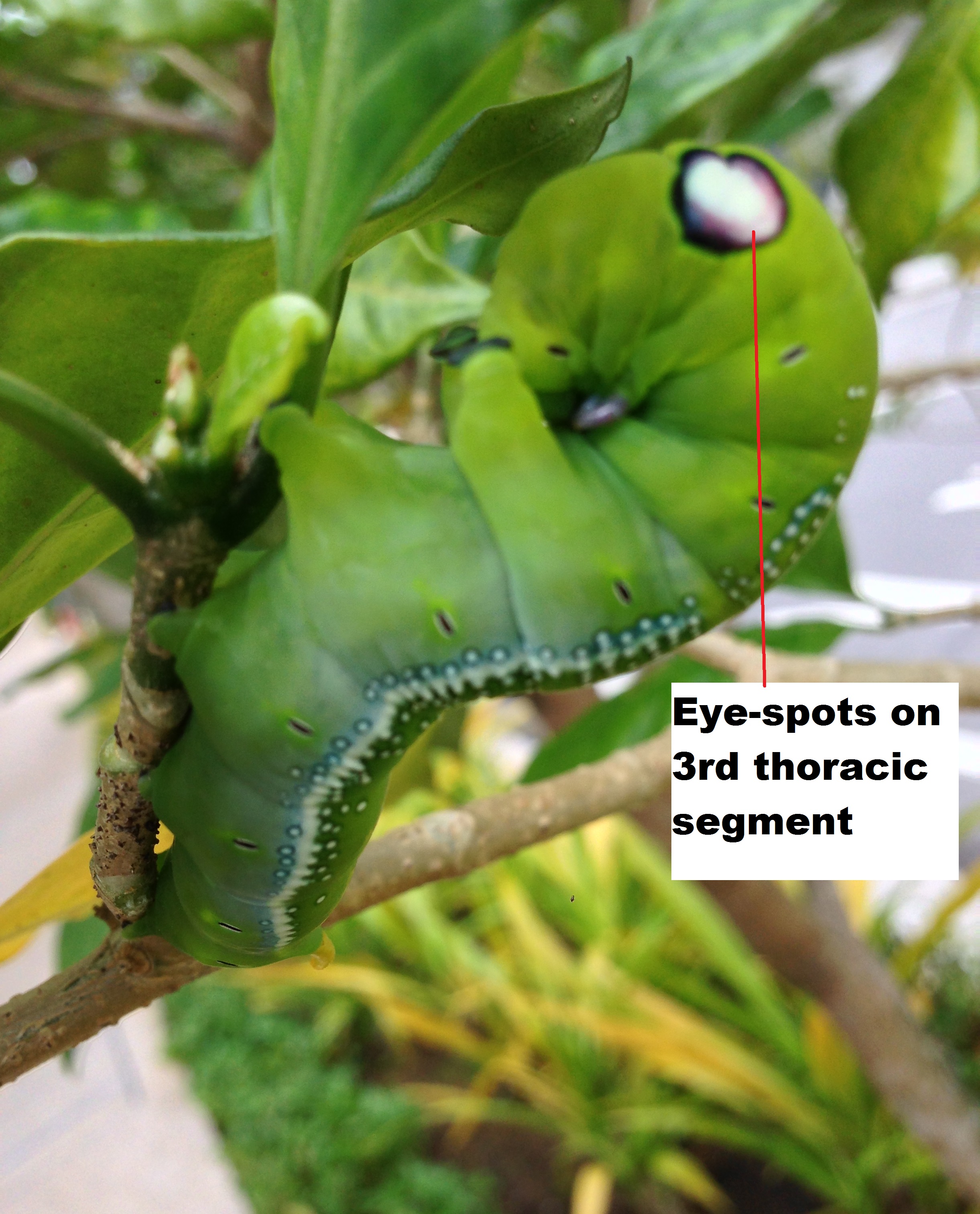

Fig. 3. Display of eye-spots in green phase of last instar (left) (photograph by Pearlynn Sim) and in the brown phase (right) (photograph by Sabrina Tang).
While the larvae of D. nerii are able to pupate in almost any environment, they sometimes build a cocoon of leaves to disguise and protect themselves before they enter the vulnerable pupa stage.
Video 2. Final instar larva of D. nerii building a cocoon out of the leaves of a Jasminum plant
One complete lifecycle from the time of hatching to egg-laying takes approximately 28 days to upwards of 30 days.
Ø Reproduction
Daphnis nerii is only competent to reproduce after the final moult. After eclosion, the female of the species extrudes the pheromone gland for up to two days after eclosion with regular pulsations as the ova mature (Leong, 2011). Pairing usually lasts not more than four hours [4].Video 3. Eclosion of D. nerii and mating behaviour (at 2:10) . (by ririlaphoto on YouTube)
Ø Habitat
Daphnis nerii are found mostly where host plants are found, but adults are polyphagus and highly mobile, allowing them to live in most habitats, including vegetated areas such as forests, and urbanised areas with the ornamental plant hosts. Their habitat range excludes desert ranges, tundra and Antarctica.Distribution:
Daphnis nerii has one of the largest distributions of any Sphingid in the world [1]. Their range spans as far west as Hawaii, and as far east as Chichijima in Japan; as far north as Finland, Sweden, and Norway, and as far south as South Africa [4]. They are found throughout Europe, South Asia, Southeast Asia and Sub-Saharan Africa, with less concentrated ranges in the Middle East and Far East.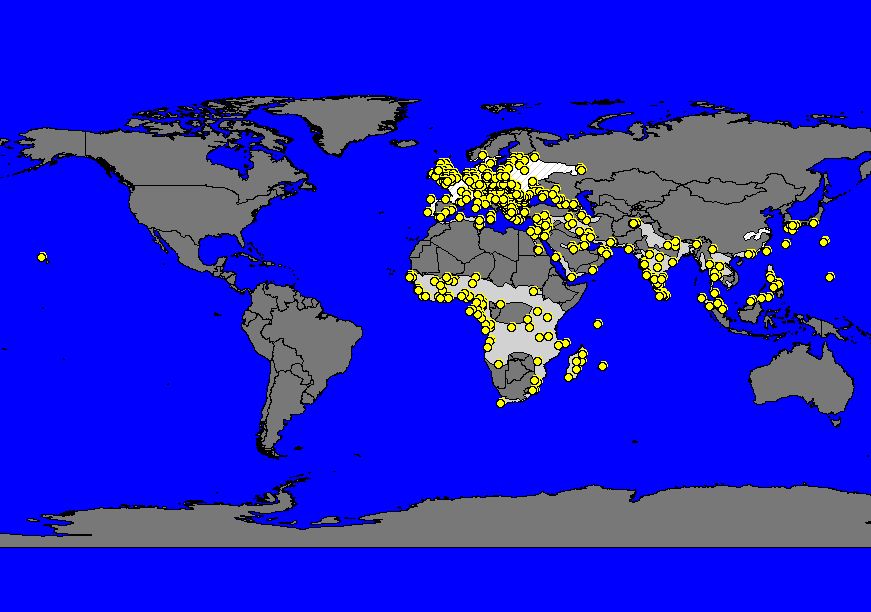
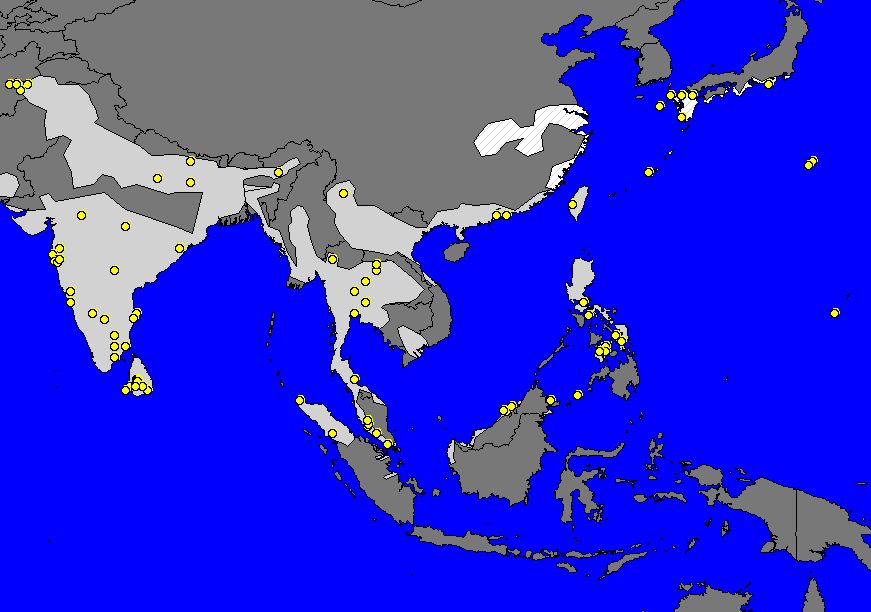
Map. 1. Map of the worldwide distribution of D. nerii (top) and of its distribution in Southeast Asia (bottom) (Beck & Kitching, 2004-2008).
The range of D. nerii is suspected to have expanded with the cultivation of N. oleander as an ornamental plant into Southeast Asia. However, the species may be more pervasive than suggested by the domestic nature of the larval host plant as D. nerii is likely non-phototaxic or negatively phototaxic, and hence may be under-recorded by light trapping methods [7].
In Singapore, the caterpillars of the moth are spotted more often than the adults, which are not attracted to light. Hence it may be easier to observe the immature larvae that have more limited mobility and do not actively avoid lit areas. Map 2 below shows recorded locations for the larvae of D. nerii. These locations are, from east to west, East Coast Terrace, Terang Bulan Ave, Yishun Ave 11, Mt Imbiah, Ngee Ann Polytechnic, and College Ave West.
Map 2. Recorded locations of D. nerii larvae in Singapore (Leong & D'Rozario, 2009), (Leong, 2011)
Diagnosis:
Although adults are generally used as standard type references, it is important when identifying this species, to be familiar with each stage of the lifecycle. The descriptions taken from Pittaway (1997-2013) are as follows:Imago (final moult): Adults generally reach a wingspan of about 10 cm. At rest, the head tends to be tucked in
and wings are held dorso-ventrally horizontal, angled towards the posterior, but not folded over the abdomen. The adults have wings with entire edges and a pointed apex on the forewing. The wings and body are usually green, pink, white and grey on the dorsal side with a smooth, banded pattern of curved lines.
| Photograph by Melissa Teo |
| Photograph by Melissa Teo |
Fig. 4. Daphnis nerii with head tucked in and wings horizontal at rest lateral (left) and dorsal (right).
The eyes are large and lack pendant lashes. Its labial palps are large and with smooth scales. The first tergite is relatively large, and weak, elongated spines occur in several rows on the abdomen. The M2 vein in the hindwing is before the centre of the cell. A basal comb is present on the midtarsus and two pairs of spurs occur on the hind tibiae with the pair closer to the body longer than the distal pair. None of the tibiae have spines.
The antennae of the male and female are slightly different with the female’s having mildly clubbed tip, and the male having bristle-like (setiform) antennae.

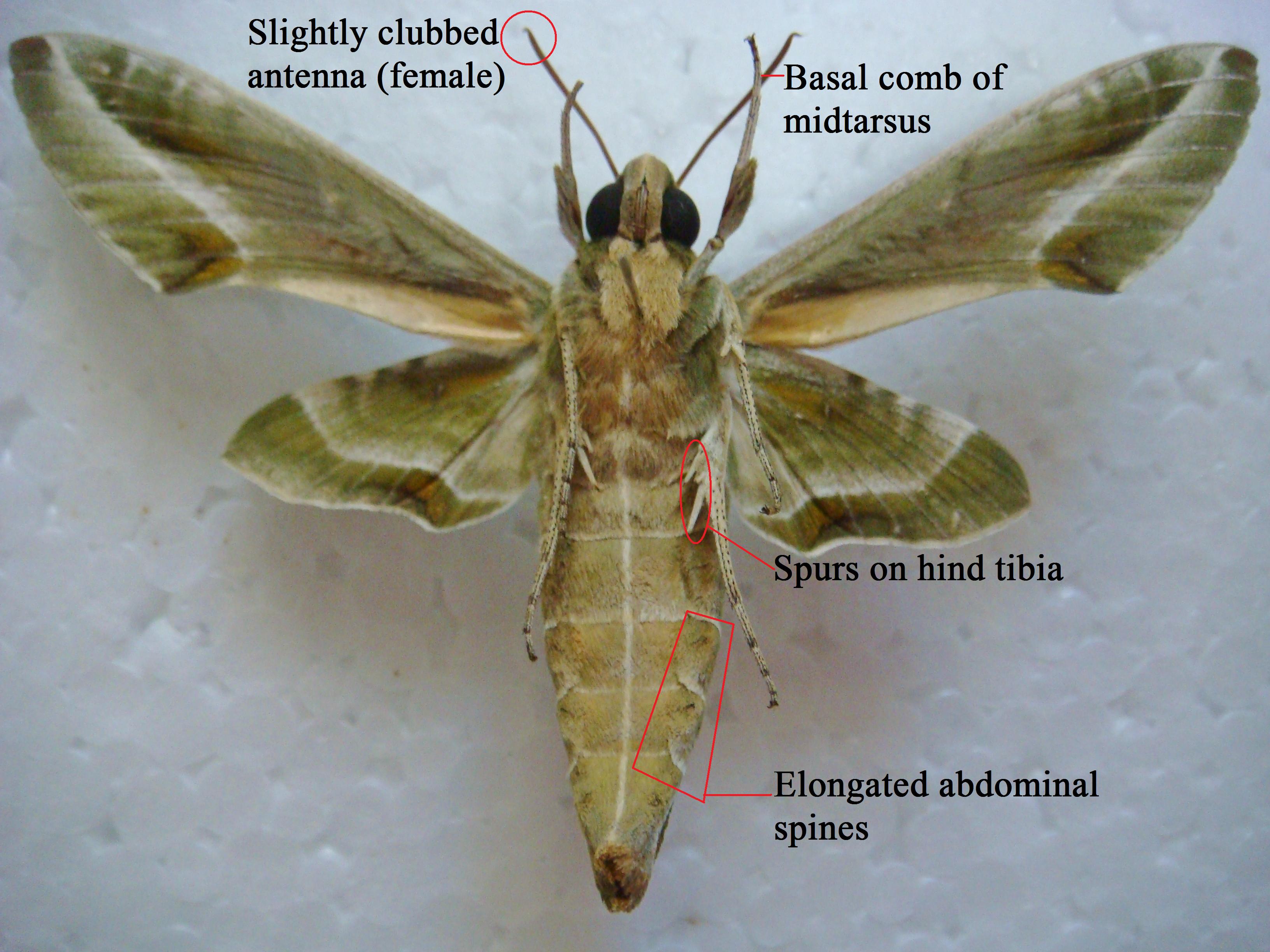
Fig. 5. Female specimen – external anatomy (information from Pittaway, 1997-2013) (Photograph by Melissa Teo)
 |
| © Photograph by A. R. Pittaway |
 |
| Photograph by Melissa Teo |
Fig. 6. Comparison of distinguishing external morphology of male and female specimens.
- Genitalia.
Males have valva with fewer than ten modified friction scales on the outer surface. The sacculus has a proximal and a distal dorsal process and the aedeagus can have one or two processes on the left, but a longer one on the right. Females have a lamella postvaginalis that is narrowed suddenly and concaved at the end. The apical margin of the structure is raised and projected towards the ventral side.
Table 1. Diagnostic description of juvenile life stages [4]
| Ovum: The eggs of D. nerii are almost spherical and about 1.5 mm in diameter. They are generally pale green in colour with a smooth chorion that is shiny. |
|
||||
| Larva (first instar): The caterpillars are about 3 to 4 mm in length when they first hatch and are bright yellow in colour. They possess a long, thin, black horn on their posterior which tapers towards the end. |
|
||||
| Larva (second instar): After the first moult, a pair of dorso-lateral lines start to appear on the first abdominal segment to the last. The larva assumes an apple-green colour and the tail horn gains a white tip. Eye-spots start to appear on the third thoracic segment. |
|
||||
| Larva (third instar): The eye-spots become more pronounced and the white lateral lines separate into circles with a pale blue ring with a white centre,outlined by black. The spiracles become an obvious black. |
|
||||
| Larva (fourth instar): The walking legs begin to turn pink and the tail horn becomes yellow instead of black. The lateral lines seem to consist a more dorsal band of yellow and below that a band of pale blue with the ringed circles as in earlier instars. |
|
||||
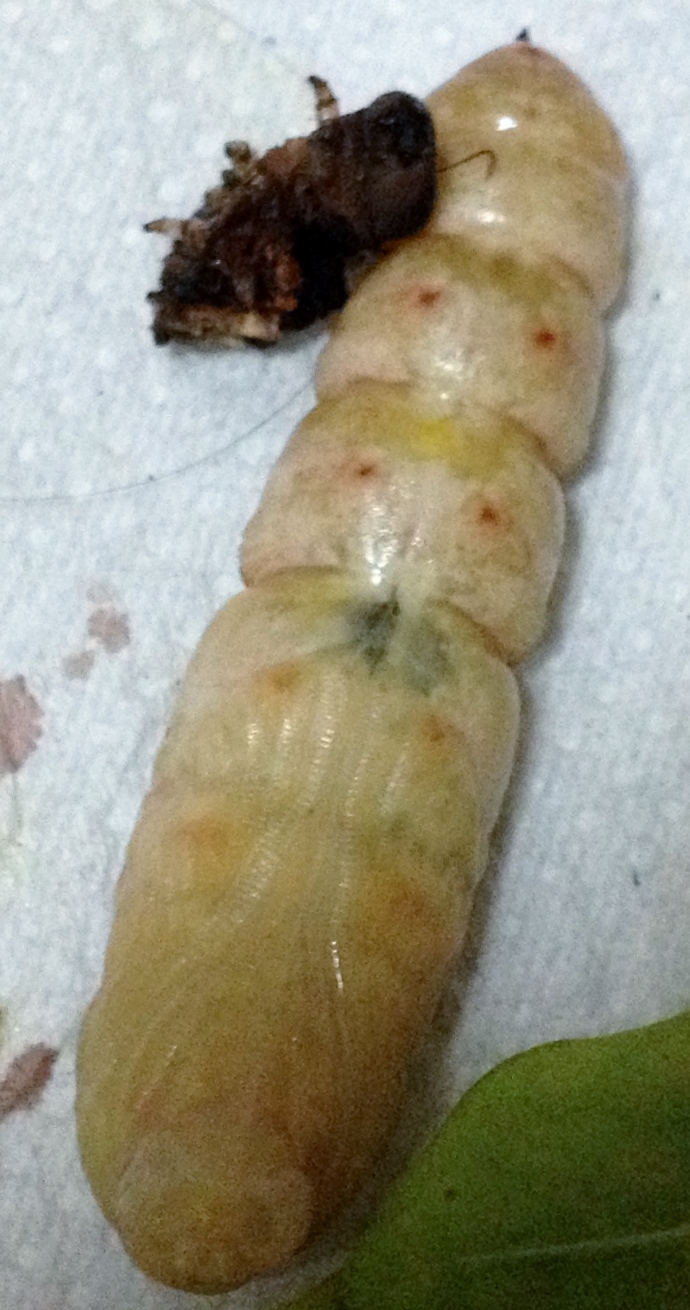
| Larva (final instar): The tapered horn becomes rounded and bulbous with bright yellow colouration. As it comes closer to pupating, the larva changes from green to brown as shown on the right. The white spots of the dorso-lateral line remain as in the earlier instars |
|
||||
| Pupa: Pupae are cream coloured when freshly moulted, but eventually darken as they harden to a light wood brown. They are generally about 60 to 75 mm in length with a prominent black line bisecting the ventral head and thorax region which demarcates the future proboscis. The caterpillars sometimes make cocoons out of leaves before pupating. |
|
Taxonomy:
Ø Classification
AnimaliaArthropoda
Insecta
Lepidoptera
Sphingidae
Macroglossinae*
Macroglossini
Daphnis
nerii
*Macroglossinae is not to be confused with the taxon of the same name under Pteropodidae (which are fruit bats).
Ø Phylogeny
Kawahara et. al. (2009) have constructed a phylogenetic framework for Sphingidae by way of a large scale molecular analysis of hawkmoth phylogeny based on 131 species as morphology has been unable to confidently determine relationships. Five protein-coding nuclear genes were analysed and both maximum likelihood and maximum parsimony analyses were used to construct the phylogeny within the Sphingid taxon.
Previous analyses have shown strong support for the monophyly of Sphingidae, and the placement of this taxon in Bombycoidea, but not for the clades within. [8] Hence, the following phylogenetic tree was produced to improve the resolution within the clade.
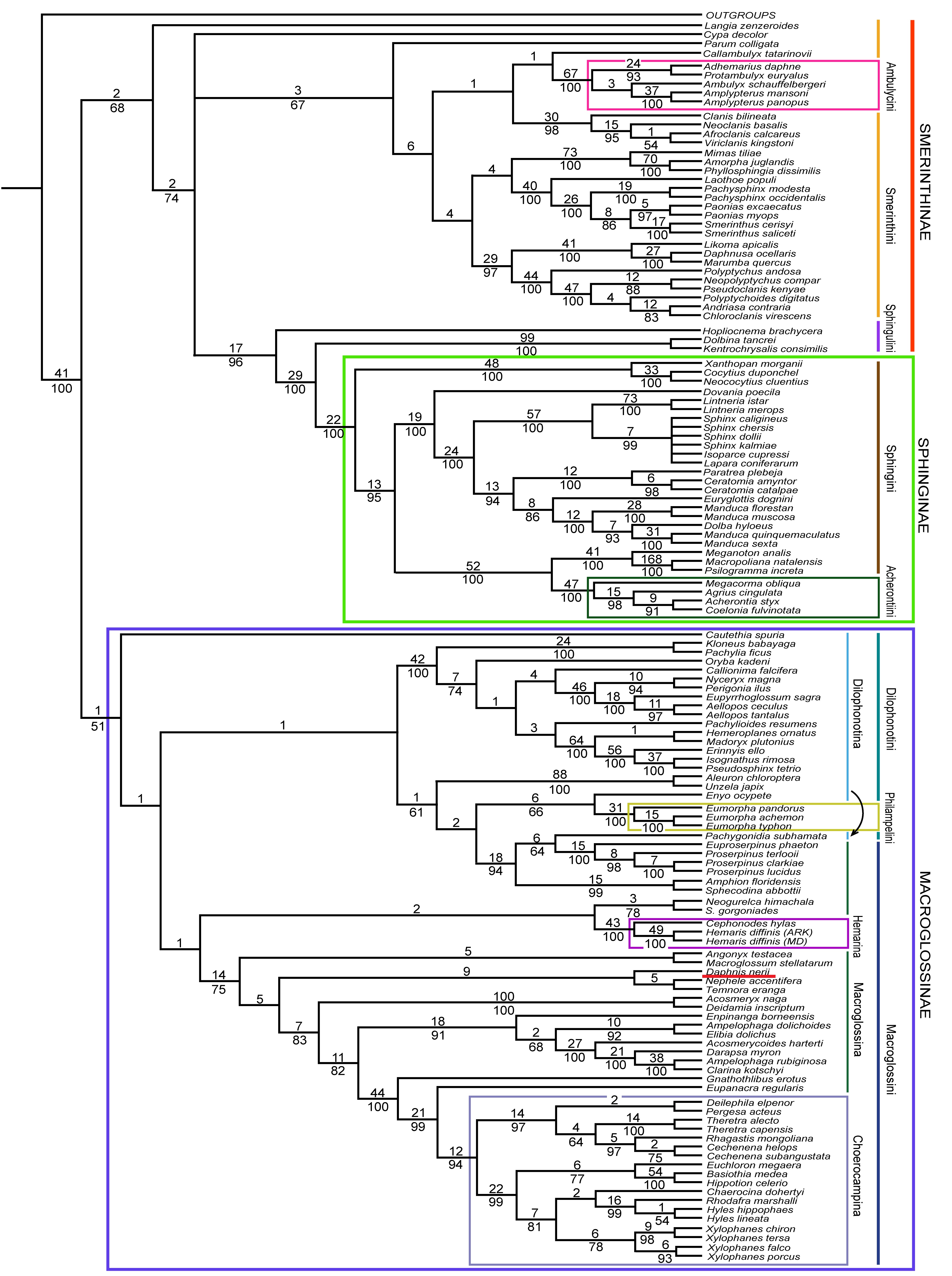
Fig 7. Phylogenetic tree of maximum parsimony (including D. nerii marked out in red) [8]
This particular phylogenetic tree was constructed using 2929 bp of CAD, 1283 bp of DDC, 1228 bp of EF-1α,951 bp of period, and 403 bp of wingless. Although the focus of the study was on the model of maximum likelihood, maximum parsimony trees were of similar topology with strong bootstrap support each. [8] Of particular note is Macroglossinae* to which D. nerii belongs. This molecular analysis has strongly corroborated the previous hypothesis for the monophyly of Macroglossinae* with a bootstrap percentage of 91% under the maximum likelihood model. The maximum parsimony model did not receive as much support, but it had overall weaker support as well.
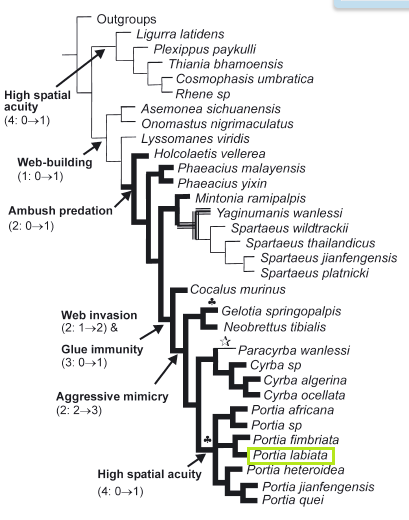
Fig 8. Phylogenetic tree of maximum likelihood for Macroglossinae* (including D. nerii marked out in red) [8]
Glossary of Terms:
Chorion is the equivalent of an egg shell in insects.
Eclosion refers to the process in which a pupa emerges as an adult insect.
Holometabolous refers to insects that undergo complete metamorphosis to become mature adults and larvae are significantly different from the adults, with a different morphology and often separate food source.
Host plant refers to the only species, or one of a few related species of plant that a caterpillar will feed on if it is host specific.
Morphology refers to the form and structure of an organism or its features.
Phototaxic refers to the type of organisms that respond to light stimulus. Positive phototaxy means the organisms reacts to light stimulus by approaching the source, and negative phototaxy means the organisms “run away” from light sources.
Polyphagus refers to the ability to feed on many kinds of food. In this case, D. nerii is able to drink from most nectar producing flowers.
Sessile organisms are permanently attached to a substrate.
Spiracles are the external structures for gaseous exchange in insects.
Links:
CATE: http://www.cate-sphingidae.org/taxonomy/Daphnis/nerii.html
Sphingidae of the Western Palaearctic: tpittaway.tripod.com/sphinx
The Sphingidae of Southeast-Asia (incl. New Guinea, Bismarck and Solomon Islands): http://www.sphin-sea.unibas.ch/SphinSEA/species%20pages/Da_nerii.htm
References:
| [1] |
T. M. Leong and V. D'Rozario, “Final instar larvae and metamorphosis of the Oleander Hawkmoth, Daphnis nerii (Linnaeus) in Singapore (Lepidoptera: Sphingidae: Macroglossinae),” Nature in Singapore, vol. 2, no. 1, pp. 297-306, 2009. |
| [2] |
A. J. Atsma, “Daphnis,” 2000-2011. [Online]. Available: http://www.theoi.com/Heros/Daphnis.html. [Accessed 26 November 2013]. |
| [3] |
I. J. Kitching, M. J. Scoble, C. R. Smith, S. James, R. Young and V. Blagoderov, “Daphnis nerii,” 2012. [Online]. Available: http://www.cate-sphingidae.org/taxonomy/Daphnis/nerii.html. [Accessed 26 November 2013]. |
| [4] |
A. R. Pittaway, “Sphingidae of the Western Palaearctic,” 1997-2013. [Online]. Available: tpittaway.tripod.com/sphinx. [Accessed 9 September 2013]. |
| [5] |
Kew Royal Botanic Gardens, “Nerium oleander (oleander),” 2013. [Online]. Available: http://www.kew.org/accessibility/index.htm. [Accessed 10 November 2013]. |
| [6] |
T. M. Leong, “Observations of pupal eclosion and pheromone release in the Oleander Hawkmoth, Daphnis nerii (Linnaeus, 1758) (Lepidoptera: Sphingidae: Macroglossinae),” Nature in Singapore, vol. 4, no. 1, p. 369–375, 2011. |
| [7] |
J. Beck and I. J. Kitching, “The Sphingidae of Southeast-Asia (incl. New Guinea, Bismarck and Solomon Islands),” 2004-2008. [Online]. Available: http://www.sphin-sea.unibas.ch/SphinSEA/species%20pages/Da_nerii.htm. [Accessed 13 November 2013]. |
| [8] |
A. Y. Kawahara, A. A. Mignault, J. C. Regier, I. J. Kitching and C. Mitter, “Phylogeny and Biogeography of Hawkmoths (Lepidoptera: Sphingidae): Evidence from Five Nuclear Genes,” PLoS ONE, vol. 4, no. 5, p. e5719, 2009. |
| [9] |
Andrews Experimental Forest Long Term Ecological Research, “Lepidoptera of the Pacific Northwest,” 2011-2013. [Online]. Available: http://andrewsforest.oregonstate.edu/pubs/pdf/pub3739/pub3739_06.pdf. [Accessed 11 November 2013]. |
| [10] |
C. Linnaeus, Systema Naturae per Regna Tria Naturae, secundum Classus, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis., 10 ed., vol. I, Stockholm: Laurentii Salvii (Impensis Direct), 1758, p. 490. |
| [11] |
K. Murugan and A. George, “Feeding and nutritional influence on growth and reproduction of Daphnis nerii (Linn.) (Lepidoptera: Sphingidae),” Journal of Insect Physiology, vol. 38, no. 12, p. 961–967, 1992. |